
Due to a much larger cross section for interaction of the Ag(4 d) rather than F(2 p) electrons with X-ray radiation, the XPS trace back mostly the partial contribution from the 4 d orbitals of Ag to the total density-of-states profile.
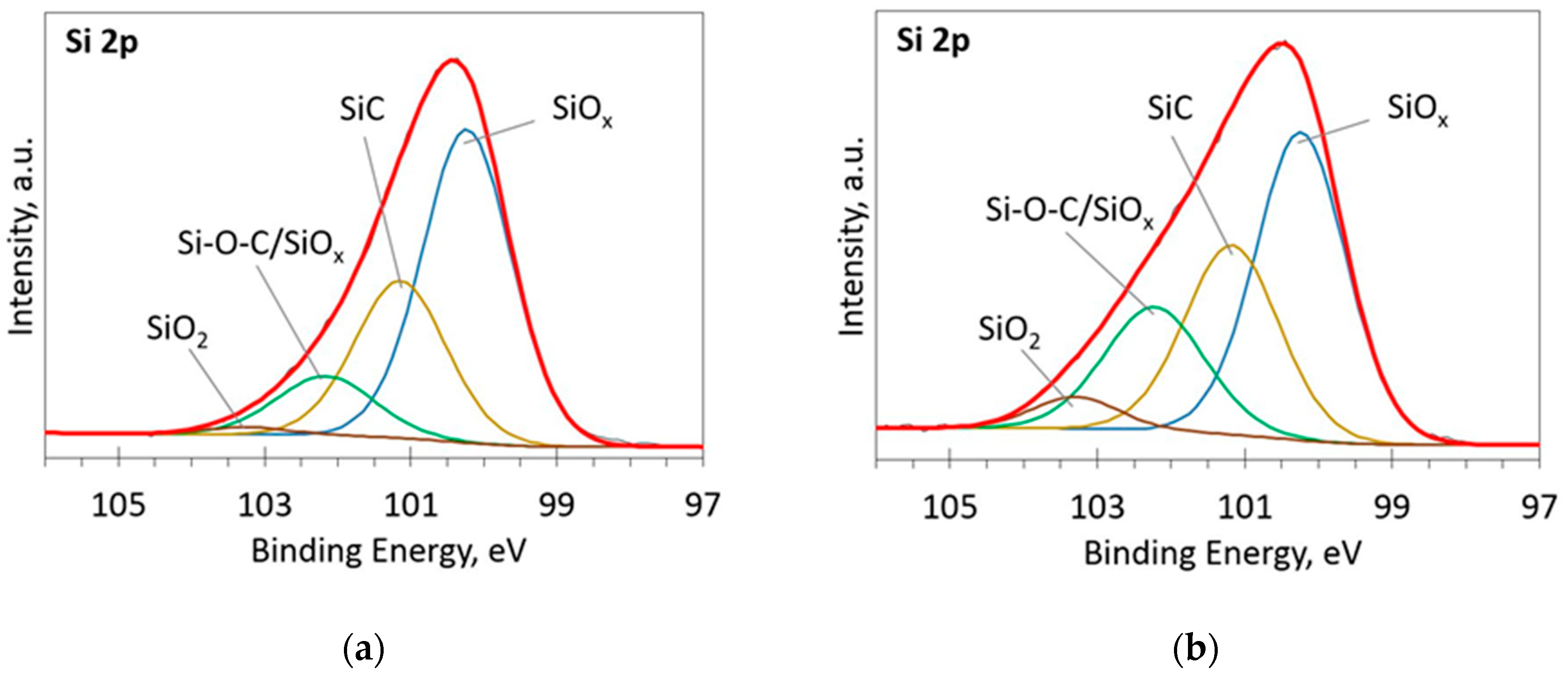
#Si2p xps peak fitting series
The X-ray photoelectron spectra (XPS) of a series of Ag 1+, Ag 2+, and Ag 3+ fluorides allow us to understand the strength of the Ag(4 d)/F(2 p) mixing taking place for AgF 2 and place it in the broader context of silver compounds at various oxidation states. Grochala, in Photonic and Electronic Properties of Fluoride Materials, 2016 5.1 M( d)/F(2 p) Mixing in MF 2 (M = Cu, Ag, Au): X-ray Photoelectron Spectra and Density Functional Theory Picture The tedious and expensive operation of XPS instrumentation hampers collecting large numbers of spectra or analysis of large sets of data when compared to other spectroscopic techniques which are more routinely available. When compared to Raman spectroscopy or extinction spectroscopy, which require only ambient conditions, XPS requires a high vacuum state, and thus the characterization is possible only in the dry state, which may not represent the bulk sample. Oxygen-containing functional groups are sensitive to X-ray beams, and they are prone to reduction during the measurement which must also be addressed. Atmospheric hydrocarbons from solvents, pump oils, and vacuum greases containing long-chain hydrocarbons are common contaminants, especially because of their affinity towards graphene. While XPS is a sensitive surface characterization technique, contamination of the sample by hydrocarbons is the biggest challenge. (B) Identification of percentage composition of carbon bonding in the same sample C1 is C C, C2 is C O, and C3 is C O carbon species. (A) XPS spectra of graphene exfoliated in organic solvent using a kitchen blender in comparison to that of graphite, which shows the absence of significant oxidation of the sample during exfoliation. Atomic percentage values and elemental ratios were calculated from the peak-area ratios after correction with the experimentally determined sensitivity factors, being reliable within ± 10%.įigure 3.7. XPS data were also used to examine the atomic composition and surface species of initial and modified polymer. This peak-fitting was repeated until an acceptable fit was obtained. Curve fitting was performed using the nonlinear list-squares algorithm and assuming a mixed Gaussian/Lorentzian peak shape of variable proportion. Data processing was performed according to ECLIPSE program, applying Schirley-type background subtraction. Each spectral region was scanned between 10 and 20 times depending on the signal intensity. Survey scans were collected from 0 to 1200 eV with pass energy of 50 eV for each sample followed by the regions C1s, N1s, and O1s. The X-ray gun was operated at 15 kV and 20 mA. The measurements were performed at base pressure lower than 8 XPS spectra were obtained by means of ESCALAB-210 electron spectrometer (VG Scientific Ltd., Sussex, U.K.), employing mg Kα X-rays.
Goworek, in Studies in Surface Science and Catalysis, 2002 2.2.1 XPS measurements The change in chemical environment contributes to the observed shift in peak positions and intensities.Ī.

These peaks of carbon-containing oxygen for DPPES–GNO are slightly shifted from those in the spectrum of pure GNO, owing to the strong chemical interaction between DPPES molecules and GNO. 11.5(c) that correspond to C–P at 286.1 eV 28,38 and C–Si at 284.0 eV 3,46 verify the grafting reaction of DPPES on GNO. DPPES–GNO exhibits similar oxygen functionalities, but with much lower peak intensities than those of GNO. 11.5(b) shows significantly stronger signals than that of GNs, indicating effective oxidation of GNs after the chemical reaction. The main peaks of C1s at 284.3 and 284.7 eV are attributed to the sp2 carbon of C C bonding and the sp3 carbon of C–C in the graphitic structure, whereas the four peaks at 288.5, 287.1, 286.5, and 285.3 eV are attributed to the carbon atoms in the O–C O, C O, C–O–C, and C–OH functional groups, respectively, 15,41,47 as displayed in Fig. The C1s spectrum of GNs includes three peaks that are attributed to the oxygen-containing functional groups, hydroxyl, epoxy, and carbonyl.

11.5 displays the C1s peaks of GNs, GNO, and DPPES–GNO. It can provide useful information on the nature of the functional groups and the functionalization of GNO. XPS is a method for analyzing the surface of a material.


 0 kommentar(er)
0 kommentar(er)
